Short Overview of Final Results:
From the very beginning of the project the consortium had a clear goal in mind reflected by the project’s title: “Tobacco as sustainable production platform of the natural biopolymer cyanophycin as co-product to oil and protein” This concept couldn’t come at a better time. Tobacco is mostly known as a smoking plant and the World Health Organisation (WHO) prompts farmers to rethink the agricultural utilisation of tobacco, as the global smoking industry is declining. This project would allow tobacco to serve as a source for biofuel, biomaterials and a food/feed protein.
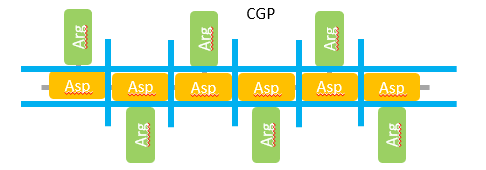
To achieve this pioneering goal a combination of plant and industrial biotechnology was used to produce cyanophycin (CGP) in the tobacco plants. Two different commercial tobacco varieties were compared to identify the best producer line. This was done by cultivating several events in a greenhouse. After identifying the best performers and homozygous lines, six events of each variety were selected to be grown during the first field trial. The plants were grown and harvested in a field in Argentina. After analysing the additional data gathered from the first field trial the best events were selected again and planted for a second field trial. Finally, the data from that trial allowed the selection for the third and final field trial. The material produced in the greenhouse and field trials was also used to research the most promising ways to isolate and use the CGP in the plants. For this, three main objectives were pursued:
- Identifying ways to extract the CGP from silage or dried leaves instead of freeze-dried material
- Minimizing the cost and steps at pH1 in the isolation procedure
- Identifying new CGP products
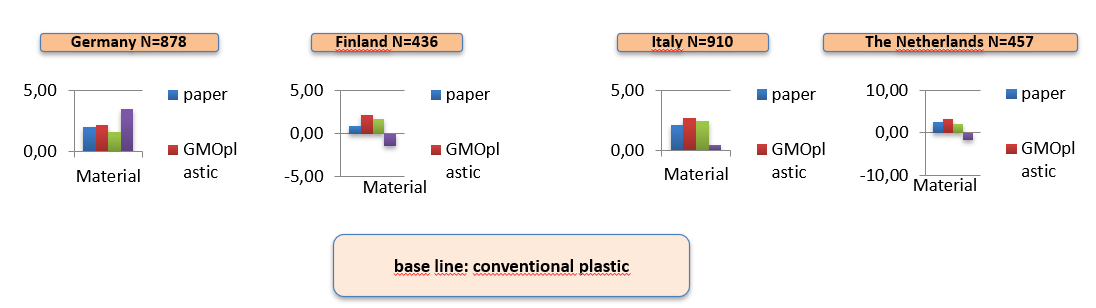
The data gathered in these steps was used to calculate the cost and benefits from the CGP production in plants and see if this would be economically feasible as well. At the same time stakeholder interviews were performed in Argentina and a consumer choice study was conducted in Germany, Italy, Finland and the Netherlands to explore, if the public would accept the new technology and potential products. We conducted conjoint-based experiments for wrapping materials and cosmetics in order to research the acceptance of potentail products made by GMO based materials.
Finally, the project also kept in mind the impact of the project for the responsible research and innovation mission. To ensure best practices we made it a priority to actively engage with internal and external stakeholders, not letting the challenges of the pandemic get in the way of that. Given that the researchers were an international consortium, with a balanced ratio of men and women in project management and teams, regular online meetings were scheduled anyway. Despite the pandemic there was personal exchange between the project partners from Wageningen, Rostock, Rosario and Lüneburg. Online platforms like Fairdom were used to exchange data and results in between those meetings. A bi-lingual ethnography researcher from Rio de la Plate was present in the meetings and the field work. She interviewed different local stakeholder groups in Argentina.
Although the results from the final field trial were delayed because of the pandemic, we have identified the elite events and the optimal cultivar. Thus, we have come quite far regarding implementation of tobacco as a production platform. When the elite events are verified by the third field trial, they can be directly used to create homogenous seed stocks for the commercial large scale cultivation of CGP tobacco. Regarding the infrastructure for large scale production the facility in Argentina has already reached a technology readiness level (TLR) of 4 for the CGP isolation process.
Multiple materials have been identified as potential end products which can be produced from CGP. This process was not without challenges. For use of CGP and poly-aspartate, with various degree of lysine or ornithine inclusions, in novel applications such as pH-tunable polymers, it has taken longer than expected to get purified fractions. But with the third field trial sufficient CGP can now be obtained in the required purity to perform various application tests. It has also been confirmed that CGP and its partial hydrolysis derivatives can’t be used for preservative applications as they show too little solubility and/or antimicrobial activity.
Transgenic tobacco events in two commercial varieties have been produced. In the greenhouse up to 11% of dry weight was CGP, which was reduced to 4% in the field. The potential elite events can produce more than 3g CGP/plant in the field without a yield penalty even under non-optimal conditions. Three events across the three field trials even produce 4g CGP/plant without a significant yield penalty. A lot of experience on growing CGP-tobacco in the field has been gained and the best cultivar was identified. The pilot plant in Argentina is established and can be adapted to the cheaper and easier method to isolate CGP, that has been discovered during the research. This method can also be adapted to industrial production in mid-scale. The research also identified several possible applications for CGP. Results from the consumer choice study and the stakeholder interviews in Europe and Argentina gave many useful insights into the public acceptance of cultivating CGP tobacco and it seems like there is a high public acceptance for CGP derived products. The consumer choice study even showed a high acceptance of CGP-based cosmetics and packaging.
Finally, a means to calculate the market potential of the plant made CGP has been established. Of course, there were challenges along the way and the work was strongly hindered by the pandemic but we are grateful for being able to continue and finish the project successfully. Overall, the international collaboration was essential for the success of the project, even though the regulations of plant material transfer between the continents proofed a great challenge.